Products
Biochemistry & Immunology
ELISA Kits
Mouse
SKU :
Option name 1
Categories : 1. Chemical and Reagents , Biochemicals , ELISA Kits & Assay Kits , Servicebio ,
Brand : Servicebio
Share
Product Information
Product Name | Cat. No. | Spec. |
Mouse TNF-alpha ELISA Kit | GEM0004-48T | 48T |
GEM0004-96T | 96T |
Product Description
TNF alpha is a multifunctional proinflammatory cytokine that belongs to the tumor necrosis factor (TNF) superfamily. This cytokine is mainly secreted by macrophage and bind to its receptors, TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR. TNF alpha is involved in the regulation of immune cells, cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation. Other functions of TNF-alpha include its role in the immune response to bacterial, viral, parasitic and certain fungal infections, as well as its role in the necrosis of specific tumors. TNF alpha causes cytolysis or cytostasis of certain transformed cells, being synergistic with interferon-gamma in its cytotoxicity. This cytokine has been implicated in a variety of diseases, including autoimmune diseases, insulin resistance, and cancer. Mouse TNF-alpha ELISA Kit is a dual antibody sandwich enzyme-linked immunosorbent assay for the in vitro quantitates mouse TNF-alpha in serum, plasma, tissue homogenates, cell lysates, or cell culture medium. The assay will exclusively recognize both natural and recombinant mouse TNF-alpha.
Storage and Shipping Conditions
Ship with wet ice; Store at 4, valid for 12 months; Use within 4 weeks once opened.
Product Components
Component Number | Component | GEM0004-48T | GEM0004-96T |
GEM0004-1 | Precoated Plate | 48T | 96T |
GEM0004-2 | Standard | 1vial | 2vials |
GEM0004-3 | Detection Antibody | 60 μL | 120 μL |
G0023 | SA-HRP | 60 μL | 120 μL |
G0024 | Diluent A | 30 mL | 30 mL |
G0025 | Diluent B | 30 mL | 30 mL |
G0026 | TMB Substrate | 6 mL | 11 mL |
G0027 | Stop Solution | 6 mL | 6 mL |
G0028 | 25x Wash Buffer | 30 mL | 30 mL |
G6077 | Plate Sealers | 4 each | 4 each |
Manual | 1pc | 1pc | |
Additional Materials Required
1. Microtiter plate reader capable of measurement at 450nm. The reference wavelength is 630 nm (Refer to the instruction manual supplied with the instrument to pre-warm).
2. Single or multichannel pipettes and tips, loading slots, centrifuge tubes.
3. Deionized or distilled water.
4. Dry filter or absorbent paper.
5. Vortex mixer, microplate oscillator.
Sample Collection And Storage Instructions
1. Serum: Collect samples to clot for 30 minutes at room temperature before centrifugation at 1000 x g for 15 minutes at 2-8°C. Collect the supernatant to carry out the assay, make aliquots and store at -20 to avoid repeated freez-thaw cycles.
2. Plasma: Collect plasma with EDTA, sodium citrate or heparin as anticoagulants, and centrifuge at 1000 x g for 15 minutes at 2-8°C within 30 minutes of collection, aspirate supernatant for testing, make aliquots and store at -20°C to avoid repeated freez-thaw cycles.
3. Tissue homogenates: The tissues should be rinsed in pre-cold PBS to remove excess blood thoroughly. Weigh and mince into small pieces in homogenizer on ice. The homogenate was homogenised by adding 10 times the weight of the tissue in PBS and sonicated until clarified. Centrifuge at 12,000 x g for 5 minutes, discard the precipitate and aspirate supernatant to test, make aliquots and store at -20°C to avoid repeated freez-thaw cycles.
4. Cell lysates
a) For adherent cells: Wash the cells with PBS 2-3 times and aspirate the residual liquid thoroughly. Aspirate cell lysate at a ratio of 250 μL lysate per well of cells in 6-well plates and flasks, and shake repeatedly to bring the lysate into full contact with the cells for 3-5 minutes. Scrape off the cells with a cell spatula and collect in a centrifuge tube. Centrifuge at 12,000 x g for 5 minutes, aspirate supernatant to test, make aliquots and store at -20°C to avoid repeated freez-thaw cycles.
b) For suspension cells: Collect cells by centrifugation and mix with cell lysate at a ratio of 250 μL of lysate per well of a 6-well plate and shaken. Ice bath for 30 minutes, repeatedly pipetting every 10 minutes to ensure complete cell lysis. Centrifuge at 12,000 x g for 5 minutes and aspirate the supernatant to assay, make aliquots and store the supernatant at -20 to avoid repeated freez-thaw cycles.
5. Cell culture supernatant: Centrifuge at 300 x g for 10 minutes and aspirate the supernatant to assay, make aliquots and store the supernatant at -20°C to avoid repeated freez-thaw cycles.
Note
1. Centrifuge to remove precipitate if sample is cloudy, not suitable for grossly hemolyzed and lipemic samples.
2. All the above samples should be sealed and stored for no more than 1 week at 4°C, 1 month at -20°C and 2 months at -80°C.
3. Gradually equilibrate frozen samples to room temperature before beginning assay.
Reagent Preparation
1. Remove the kit and samples from the refrigerator 20 minutes in advance and equilibrate to room temperature. Remove the plates and reagents as required for the experiment and store the remaining reagents at 4.
2. 1x Wash Buffer: Pour 30 mL of the Wash Buffer Concentrate(25x) into a 1000 mL graduated cylinder. Bring to final volume of 750 mL with distilled or deionized water. Mix gently to avoid foaming. Transfer to a clean wash bottle and store at 2-25.
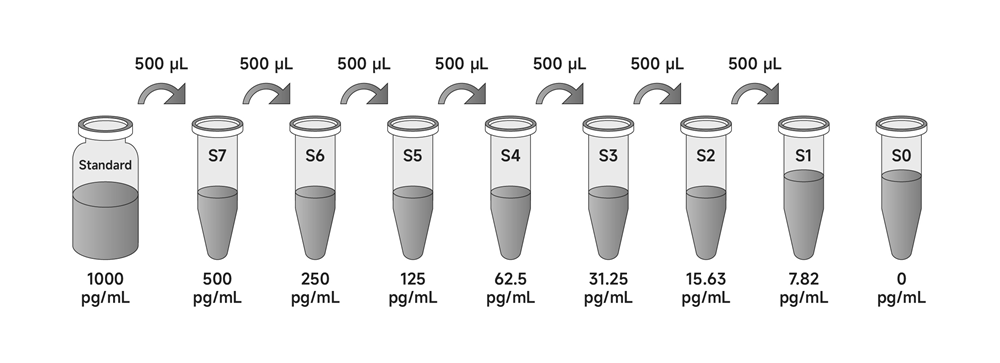
3. Standards: Add Diluent A to the standard in the labeled volume and gently vortex and shake to ensure complete mixing. The concentrated Mouse TNF-alpha Standard is obtained by redissolving the standard to a concentration of 1000 pg/mL. Allow to stand for 10 minutes after re-dissolution and mix well before dilution.
a) Preparation of standard curves for serum/plasma/tissue homogenate/cell lysate:
Mix the reconstituted standards well, add 500 µL of Dilution A to 500 µL of concentrated Mouse TNF-alpha standard as the highest concentration S7 (500 pg/mL) for the standard curve. Take six 1.5 mL centrifuge tubes (S1-S6) arrange in sequence and add 500 µL each of Dilution A. Pipette 500 µL of S7 (500 pg/mL) standard into the first tube S6, and mix gently. Pipette 500 µL from S6 into the second centrifuge tube S5 and mix with gentle blowing. And so on for multiplicative dilution of standards. S0 is diluent A.
b) Preparation of standard curve of cell culture supernatant sample:
Mix the reconstituted standards well, add 500 µL of culture supernatant to 500 µL of concentrated Mouse TNF-alpha standard as the highest concentration S7 (500 pg/mL) for the standard curve. Take six 1.5 mL centrifuge tubes (S1-S6) arrange in sequence and add 500 µL each of culture supernatant. Pipette 500 µL of S7 (500 pg/mL) standard into the first tube S6, and mix gently. Pipette 500 µL from S6 into the second centrifuge tube S5 and mix with gentle blowing. And so on for multiplicative dilution of standards. S0 is culture supernatant.
4. 1x detection antibody: Centrifuge briefly and make a 1:100 dilution of detection antibody with Diluent B to a 1x detection antibody working solution prior to use.
5. 1×SA-HRP: Centrifuge briefly and make a 1:100 dilution of SA-HRP with Diluent B to a 1x SA-HRP working solution prior to use.
Note
1. Unopened kits: Store entirely at 4°C and valid for 12 months.
2. Opened kits: Lyophilised standards should not be reused after dissolution and remaining reagents should be stored promptly at 4°C and valid for 4 weeks. In addition, keep unused slats in an aluminium foil bag containing desiccant seal tightly and store at 4°C.
Assay Protocol
1. Add sample: Set up the standard wells, sample wells and blank wells separately. Dilute the standards and samples with Diluent A. Set up 7 standard wells (S1-S7), add 100 μL of standards of different concentrations to each well, add 100 μL of Diluent A to the blank wells, and 100 μL of the sample to be tested to the remaining wells, cover the plate with a plate sealer, shake at 100-300 rpm (to ensure that the solution does not spill out of each well and can be mixed well), incubate and shake at room temperature for 2 hours.
Note: Refer to the relevant literature to determine the approximate concentration of the protein to be tested in the sample. If the concentration is greater or less than the maximum or minimum standard concentration of the kit, dilute or concentrate it appropriately before testing.
2. Plate washing: Automatic or manual plate washer with 300 μL of wash buffer per well, the interval between injection and aspiration is 15-30 seconds and wash 5 times. After the last washing, the plate is inverted and patted dry on absorbent paper with appropriate force and the liquid in the wells is discarded.
3. Add Detection Antibody: Dilute the detection antibody to working concentration with Diluent B, add 1x detection antibody working solution 100 μL per well (prepared before use), seal the plate with a new sealing film, shake at 100-300 rpm (to ensure that the solution does not spill out of each well and is well mixed) and incubate for 1 hour at room temperature with shaking.
4. Plate washing: Repeat step 2.
5. Add SA-HRP: Dilute SA-HRP to working concentration with Diluent B. Add 100 μL of 1x SA-HRP working solution (prepared before use) to each well, cover the plate with a new plate sealer, shake at 100-300 rpm (to ensure that the solution does not spill out of each well and that it is well mixed) and incubate for 30 minutes at room temperature.
6. Plate washing: Repeat step 2.
7. Add TMB Substrate: Add 90 μL of TMB substrate to each well, replace with a new plate sealer and develop the colour at room temperature protected from light (the reaction time should be controlled for 10-30 minutes. Terminate when the first 3-4 wells of the standard have a clear gradient of blue and the last 3-4 wells have no clear gradient).
8. Add Stop Solution: The reaction is terminated by adding 50 μL of stop solution to each well, at which point the blue colour immediately turns yellow. The stop solution should be added in the same order as the substrate solution as far as possible. In case of uneven colour, shake the plate gently to mix the solution well.
9. Reading: After ensuring that the bottom of the plate is free of water droplets and the wells are free of air bubbles, reading absorbance at 450 nm within 10 minutes. It is recommended to use dual wavelengths. Use 450 nm as detection wavelength, 630 nm as reference and calibration wavelength. Measure at 450 nm only will reduce accuracy.
Note
1. Store kits according to instructions. Gradually equilibrate samples to room temperature for 20-30 minutes before beginning assay.
2. The standards are lyophilised powders. The diluent volume and ratio should strictly follow the kit instructions. To ensure the accuracy of the standards, please do not reuse if they are left over.
3. The 25x Wash Buffer may crystallise at low temperatures. If crystals have formed in the Buffer Concentrates, warm them gently until they have completely dissolved before prepare to working solution.
4. TMB is Hazardous. Care should be taken to avoid contact with skin or eyes. In the case of contact with skin or eyes wash immediately with water.
5. Stop Solution contains acid, care should be taken to avoid contact with skin or eyes. In the case of contact with skin or eyes wash immediately with water.
6. An intense aqua blue color indicates that the TMB Sbustrate is contaminated, do not use it.
7. Reagents are lot-specific. Do not mix or interchange different reagent lots from various kit lots.
8. All reagents must be at room temperature (25-28). Temperature below 25will result in a significant decrease in the absorbance of the final detection.
9. Repeat wells are recommended for standards and samples to ensure confidence in the test results.
10. Do not expose kit reagents to strong light during storage or incubation.
11. Mix gently after sample addition to avoid foaming.
12. Wear suitable protective clothing such as laboratory overalls, safety glasses and gloves.
13. For research use only. Not for use in diagnostic or therapeutic procedures.
14. To avoid microbial contamination or cross-contamination of reagents or samples that may invalidate the test, use disposable pipette tips for each transfer.
15. After the last wash step, empty wells and tap microwell strips on absorbent pad or paper towel to remove excess Wash Buffers. Do not put the absorbent paper directly into the wells to absorb water.
Results Analysis
1. Results Calculation
a) To obtain more accurate results, it is recommended to calculate the average absorbance values for each set of duplicate standards and samples.
b) Create a standard curve with curve-fitting statistical software by plotting the standard concentration on the abscissa against the OD value on the ordinate. the closer the correlation coefficient R value is to 1, the better the fitting effect is. The best fitting curve was determined by regression analysis.
c) The sample concentration is calculated by substituting the OD value. If the test sample was diluted, multiply the appropriate dilution factor for actual concentration.
d) The standard curve can be linearised by taking a logarithmic fit to the concentration values and OD values. This process may result in more sample concentrations, but the accuracy of the data will be reduced.
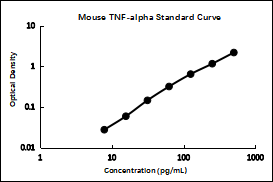
2. Typical data
Perform a standard curve with each assay. The OD values of the standard curve may vary according to the conditions of assay performance (e.g., operator, pipetting technique, washing technique, or temperature effects). The standard curves provided in the instructions are for reference only.
pg/mL | OD | Average | Corrected | |
500 | 2.210 | 2.234 | 2.222 | 2.173 |
250 | 1.195 | 1.214 | 1.205 | 1.156 |
125 | 0.681 | 0.699 | 0.690 | 0.641 |
62.5 | 0.371 | 0.362 | 0.367 | 0.318 |
31.25 | 0.189 | 0.199 | 0.194 | 0.145 |
15.63 | 0.107 | 0.109 | 0.108 | 0.059 |
7.82 | 0.072 | 0.081 | 0.077 | 0.028 |
0 | 0.048 | 0.050 | 0.049 | |
3. Sensitivity
The lower limit of detection of mouse TNF-alpha is 1.13 pg/mL (mean of 3 independent assays).
This was determined by adding two standard deviations to the mean O.D. obtained when the zero standard was assayed 20 times, and calculating the corresponding concentration.
4. Precision
Precision is expressed as the coefficient of variation(CV) of the measured values of the samples. CV(%)=(SD/Mean)×100.
Intra-assay Precision: 3 samples with low, mid and high level were assayed 20 times in multiple assays to determine precision between assays. Intra-assay Precision: CV<8%.
Inter-assay Precision: 3 samples with low, mid and high level were assayed in replicates of 8 to determine precision within an assay. Inter-assay Precision: CV<10%.
5. Spike recovery
The spike recovery was evaluated by spiking 5 levels of mouse TNF-alpha into serum. repeat the measurement and calculate the mean. The unspiked serum was used as blank in these experiments and the recovery was calculated (recovery is the ratio of the assay value to the expected value). The recoveries range from 88% to 109%, with a mean recovery of 95%.
6. Linearity of Dilution
Linearity of dilution was determined by serially diluting sample in the kinetic range of the standard curve after spiking 5 levels of mouse TNF-alpha into serum. The linearity range is the ratio of the assay value to the expected value of mouse TNF-alpha in the diluted sample.
Dilution | Mean(%) | Range(%) |
1:2 | 93 | 83-98 |
1:4 | 94 | 82-101 |
1:8 | 98 | 94-106 |
1:16 | 102 | 99-110 |
7. Sample values
The kit was applied to 30 normal mouse serum samples and all samples were below the lower limit of the detection of the standards.
8. Specificity
This ELISA kit is specific for the measurement of natural and recombinant Mouse TNF-alpha. It does not cross-react with recombinant human TNF-alpha, rat IL-6, mouse IL-6, or mouse IFN-gamma. It is not possible to perform all cross-reactivities due to the limitation of technical and sample source. There is a risk that the kit may cross-react with other substances that have not been tested.
Test Protocol Summary
1. Prepare standards, reagents and samples according to instructions before the experiment.
2. Add 100 μL of sample (standard or sample) to all wells and shake for 2 hours at room temperature.
3. Wash 5 times and pat dry, add 100 μL of detection antibody working solution to all wells and shake for 1 hour at room temperature.
4. Wash 5 times and pat dry, add 100 μL of SA-HRP working solution to all wells and shake for 30 minutes at room temperature.
5. Wash 5 times and pat dry, add 90 μL of TMB substrate to all wells and incubate for 10-30 minutes at room temperature in the dark.
6. Add 50 μL of stop solution to all wells.
7. Read absorbance at 450 nm within 10 minutes, optionally 630 nm as the reference wavelength.
Layout
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
A | S7 | S7 | ||||||||||
B | S6 | S6 | ||||||||||
C | S5 | S5 | ||||||||||
D | S4 | S4 | ||||||||||
E | S3 | S3 | ||||||||||
F | S2 | S2 | ||||||||||
G | S1 | S1 | ||||||||||
H | S0 | S0 |
For Research Use Only!